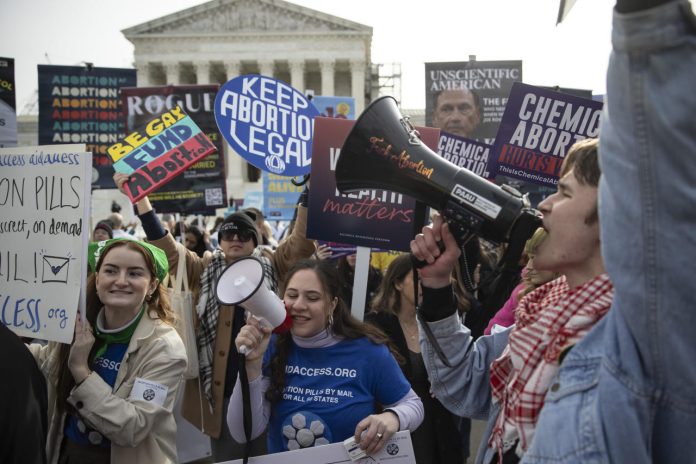The Supreme Court on Thursday dismissed a challenge against the availability of the widely used abortion pill mifepristone, maintaining access to the drug. This marks the court’s first significant ruling on abortion since the reversal of Roe v. Wade.
The justices unanimously ruled on procedural grounds, determining that the group of anti-abortion rights doctors and medical associations who sued the Food and Drug Administration (FDA) lacked the legal right to do so.
By finding that the plaintiffs did not have legal standing, the Supreme Court avoided addressing whether the FDA acted lawfully in 2016 and 2021 when it eased restrictions on mifepristone.
“Under Article III of the Constitution, a plaintiff’s desire to make a drug less available for others does not establish standing to sue. Nor do the plaintiffs’ other standing theories suffice,” Justice Brett Kavanaugh wrote for the court. “Therefore, the plaintiffs lack standing to challenge FDA’s actions.”
The decision does not prevent other challenges against mifepristone, but it means the FDA’s recent measures to make the drug more accessible will remain. These measures include allowing mifepristone to be taken later into pregnancy, expanding the range of healthcare providers who can prescribe it, and lifting the in-person dispensing requirement so it can be mailed.
Three states—Idaho, Kansas, and Missouri—attempted to intervene in the case but were denied by the Supreme Court. However, a federal district judge allowed them to join the suit at the district court level. Erin Hawley, a lawyer for the Alliance Defending Freedom representing the doctors, indicated that litigation would continue with these states presenting different standing arguments.
The lawsuit, filed by anti-abortion rights doctors and medical associations in November 2022, challenged the FDA’s authority to approve mifepristone and questioned the drug’s safety and effectiveness. The plaintiffs targeted the FDA’s 2000 approval of mifepristone and the 2016 and 2021 actions that increased its accessibility.
Mifepristone, used in conjunction with misoprostol to end early pregnancies, has been taken by over 5 million patients since its FDA approval in 2000, with serious adverse events being exceedingly rare, according to the FDA. In 2023, medication abortions accounted for more than half of all abortions in the U.S. healthcare system, according to a Guttmacher Institute study.
U.S. District Judge Matthew Kacsmaryk had blocked the FDA’s 2000 approval of the drug and its subsequent actions, deeming them likely unlawful. This ruling would have removed mifepristone from the market. However, the Supreme Court preserved access to the drug during ongoing legal proceedings. A federal appeals court later partially upheld Kacsmaryk’s ruling, affirming the FDA’s approval of mifepristone but finding that the 2016 and 2021 changes likely violated the law.